Comparison Table for Bone Regeneration MaterialsBrand and Product Name Packaging Sterility Absorption Rate Ideal Use HealiAid Collagen Plug by Surgical Esthetics Individually packaged Sterile N/A Bone graft covering or socket filler OSSIF-i sem™ Mineralized Cortical-Cancellous Bone Allograft SP by Surgical Esthetics Double-sterile pack Sterile N/A Elevation of maxillary sinus, ridge augmentation, large extraction sockets, and volume maintenance OSSIF-i sem™ Demineralized Cortical Bone Allograft by Surgical Esthetics Double-sterile pack Sterile N/A Treatment of periodontal defects, filling of extraction sockets, and composite grafting HeliMend Collagen Membrane – Miltex by Integra N/A N/A 4-8 weeks Guided tissue regeneration procedures OSSIF-i sem™ Mineralized Cancellous Bone Allograft by Surgical Esthetics Double-sterile jar Sterile N/A Ridge augmentation before implant placement, periodontal defects, filling of extraction sockets, and elevation of maxillary sinus Foundation™ Bone Filling Material by J Morita USA Individually packaged in a sterile container Sterile N/A Filling extraction sockets after teeth extractions to provide support for implants, bridges, and dentures R.T.R. Membrane by Septodont Inc N/A N/A N/A Compatible with RTR bone regeneration products CollaShield™ Collagen Membrane by Surgical Esthetics Double-sterile pack Sterile N/A Guided bone and tissue regeneration HeliMend Advanced Collagen Membrane by Miltex by Integra in the form of a collagen membrane Sterile 18 weeks It is used during guided tissue regeneration procedures where a longer absorption rate is desired. R.T.R.+ Synthetic Biphasic Bone Grafting Substitute Syringe by Septodont Inc syringe form with a volume of 0.5 cc. Sterile The absorption rate depends on the composition It is used for reconstructing bone gaps or bony loss in maxillofacial applications
Top 10 Bone Management Products for Dentists
1. HealiAid Collagen Plug by Surgical Esthetics

Product Description
HealiAid Collagen Plug by Surgical Esthetics is a sterile, absorbable collagen wound dressing designed for effective socket management and graft site protection. Its porous and pliable structure makes it easy to shape, cut, and apply to various surgical sites.
Product Overview
Ideal for use as a socket filler or bone graft covering, the HealiAid Collagen Plug supports wound healing by stabilizing clots and protecting exposed sites. Each plug is individually packaged, sterile, and intended for single use. Its nonpyrogenic packaging ensures safety and compliance in clinical settings.
Key Features
- Porous, absorbable collagen structure for optimal healing
- Pliable and easy to cut or mold to fit site-specific needs
- Ideal for use as socket filler or graft covering
- Sterile and individually packaged for infection control
- Single-use only
- Nonpyrogenic packaging for added safety
Common Applications
- Post-extraction socket management
- Coverage for bone grafts and surgical sites
- Periodontal and implant procedures requiring wound stabilization
Technical Specifications
- Product Type: Absorbable collagen wound dressing
- Sterility: Sterile
- Packaging: Individually packaged
- Use: Single-use only
- Material: Porous collagen
- Manufacturer: Surgical Esthetics
- Packaging Type: Nonpyrogenic
Customer Ratings
Average Rating: 4.8 out of 5 (based on clinical feedback)
Customer Feedback
- Easy to shape and place
- Excellent for clot stabilization and site protection
- Reliable healing aid in grafting and extraction procedures
- High quality and convenient individual packaging
2. OSSIF-i sem™ Mineralized Cortical-Cancellous Bone Allograft SP by Surgical Esthetics

Product Description
OSSIF-i sem™ Mineralized Cortical-Cancellous Bone Allograft SP by Surgical Esthetics is a high-quality bone graft material designed for predictable bone regeneration. This allograft combines both cortical and cancellous components to support volume stability and rapid integration in a wide range of dental surgical applications.
Product Overview
Processed to ensure safety and biocompatibility, OSSIF-i sem™ SP offers an ideal balance of structure and porosity. The cortical component provides long-term support and space maintenance, while the cancellous matrix promotes vascular infiltration and bone remodeling. It is supplied sterile and ready for immediate clinical use.
Key Features
- Combination of mineralized cortical and cancellous bone
- Enhances space maintenance and bone volume stability
- Promotes angiogenesis and osteoconduction
- Sterile and ready-to-use
- Suitable for ridge preservation, sinus lifts, and socket grafting
Common Applications
- Ridge preservation after extractions
- Periodontal defect repair
- Sinus augmentation procedures
- Implant site development and socket grafting
Technical Specifications
- Product Type: Bone allograft
- Composition: Mineralized cortical-cancellous blend
- Sterility: Sterile
- Use: Single-use, ready-to-use
- Manufacturer: Surgical Esthetics
- Packaging: SP (premeasured, sterile packaging)
Customer Ratings
Average Rating: 4.9 out of 5 (based on professional feedback)
Customer Feedback
- Excellent handling and graft stability
- Consistently high-quality results
- Great for both large and small defect repairs
- Trusted material for implant site preparation
3. OSSIF-i sem™ Demineralized Cortical Bone Allograft by Surgical Esthetics

Product Description
OSSIF-i sem™ Demineralized Cortical Bone Allograft by Surgical Esthetics is an advanced grafting material designed for regenerative dentistry and socket preservation. Featuring 0.25–1 mm particle size, this demineralized bone matrix provides both osteoinductive and osteoconductive properties to promote reliable bone regeneration.
Product Overview
Packaged in a double-sterile jar for direct transfer to the surgical field, OSSIF-i sem™ offers excellent handling once hydrated. It is ideal for periodontal defects, socket grafting, and use with implants. Each lot is tested for inductivity to ensure consistent clinical performance and biologic activity.
Key Features
- Demineralized cortical bone with 0.25–1 mm particle size
- Proven osteoinductive and osteoconductive properties
- Excellent handling characteristics upon hydration
- Packaged in double-sterile jars for direct sterile field use
- Lot-tested for inductivity and clinical consistency
Common Applications
- Periodontal defect treatment
- Extraction socket grafting
- Grafting in conjunction with implant placement
- Composite grafting with other OSSIF-i sem™ products
Technical Specifications
- Product Type: Demineralized cortical bone allograft
- Particle Size: 0.25–1 mm
- Sterility: Double-sterile packaging
- Use: Ready-to-use upon hydration
- Manufacturer: Surgical Esthetics
- Testing: Lot tested for inductivity
Customer Ratings
Average Rating: 4.9 out of 5 (based on clinician feedback)
Customer Feedback
- Excellent handling and adaptability to site
- Trusted for consistent bone regeneration
- Convenient sterile packaging for surgical environments
- Strong results in both socket and implant grafting cases
4. HeliMend Collagen Membrane – Miltex by Integra

Product Description
HeliMend™ Collagen Membrane by Miltex (Integra) is an absorbable membrane made from purified bovine tendon, designed to support guided tissue regeneration (GTR) and guided bone regeneration (GBR) procedures. It offers structural integrity and biocompatibility, making it ideal for wound stabilization and space maintenance.
Product Overview
HeliMend is engineered to provide a longer resorption time of 4–8 weeks, allowing for extended barrier function during healing. Its natural collagen composition is easily handled, pliable when hydrated, and conforms well to grafted sites. It resorbs naturally without the need for surgical removal.
Key Features
- Made from purified bovine tendon
- Absorbable in 4–8 weeks
- Maintains space and stabilizes wounds for GTR and GBR
- Biocompatible and pliable for easy placement
- No need for surgical removal
Common Applications
- Guided tissue regeneration (GTR)
- Guided bone regeneration (GBR)
- Socket preservation and ridge augmentation
- Periodontal and peri-implant defect coverage
Technical Specifications
- Product Type: Absorbable collagen membrane
- Material: Purified bovine tendon
- Absorption Time: 4–8 weeks
- Sterility: Sterile
- Manufacturer: Miltex by Integra
Customer Ratings
Average Rating: 4.8 out of 5 (based on professional feedback)
Customer Feedback
- Excellent handling and conformability
- Reliable barrier during extended healing periods
- Resorbs predictably without complications
- Preferred for regenerative procedures
5. OSSIF-i sem™ Mineralized Cancellous Bone Allograft by Surgical Esthetics

Product Description
OSSIF-i sem™ Mineralized Cancellous Bone Allograft by Surgical Esthetics is a porous, osteoconductive grafting material designed to promote complete bone regeneration. Its structure and particle composition provide optimal support for natural bone remodeling and space maintenance during healing.
Product Overview
Formulated for maximum esthetics and biological integration, OSSIF-i sem™ Mineralized Cancellous Bone supports host bone in-growth and provides an ideal scaffold for new bone formation. Its excellent space-maintaining properties make it suitable for a wide range of surgical and regenerative procedures.
Key Features
- Osteoconductive matrix encourages bone regeneration
- Porous structure supports host bone in-growth
- Ideal particle mix for handling and packing
- Provides space maintenance for optimal esthetic outcomes
- Promotes osseous integration and natural bone remodeling
Common Applications
- Ridge augmentation prior to implant placement
- Grafting in conjunction with implant surgeries
- Filling periodontal and osseous defects
- Extraction socket preservation
- Maxillary sinus elevation procedures
Technical Specifications
- Product Type: Mineralized cancellous bone allograft
- Properties: Osteoconductive, porous architecture
- Packaging: Sterile, ready-to-use
- Manufacturer: Surgical Esthetics
Customer Ratings
Average Rating: 4.9 out of 5 (based on clinical feedback)
Customer Feedback
- Excellent space maintenance and graft stability
- Easy to handle and pack
- Predictable healing and remodeling outcomes
- Ideal for implant and ridge development procedures
Top 5 Bone Graft Materials in Dentistry
6. Foundation™ Bone Filling Material by J Morita USA

Product Description
Foundation™ Bone Filling Material by J. Morita USA is a collagen-based augmentation material specifically designed to fill extraction sockets and support bone regeneration following tooth extractions. Its unique shape and handling properties make it easy to place and adapt to a variety of clinical situations.
Product Overview
Foundation™ promotes rapid bone growth, allowing for implant placement as early as 8–12 weeks post-extraction. Shaped like a bullet for simplified insertion, it can also be trimmed or contoured for an optimal fit in the socket. Each unit is individually packaged in a sterile container for convenience and infection control.
Key Features
- Collagen-based bone filling material
- Bullet-shaped for easy and secure placement
- Can be trimmed or shaped to fit specific sites
- Promotes accelerated bone growth and healing
- Suitable for implants, bridges, and denture support
- Individually packaged in sterile containers
Common Applications
- Filling extraction sockets
- Site preparation for implants, bridges, or dentures
- Post-extraction grafting to maintain ridge contour
Technical Specifications
- Product Type: Bone filling augmentation material
- Material: Collagen-based
- Shape: Bullet (trim-to-fit)
- Sterility: Individually packaged, sterile
- Healing Timeline: Implant placement possible in 8–12 weeks
- Manufacturer: J. Morita USA
Customer Ratings
Average Rating: 4.8 out of 5 (based on clinical feedback)
Customer Feedback
- Easy to place and shape
- Speeds up bone regeneration
- Convenient sterile packaging
- Effective for implant and prosthetic site preparation
7. R.T.R. Membrane by Septodont Inc
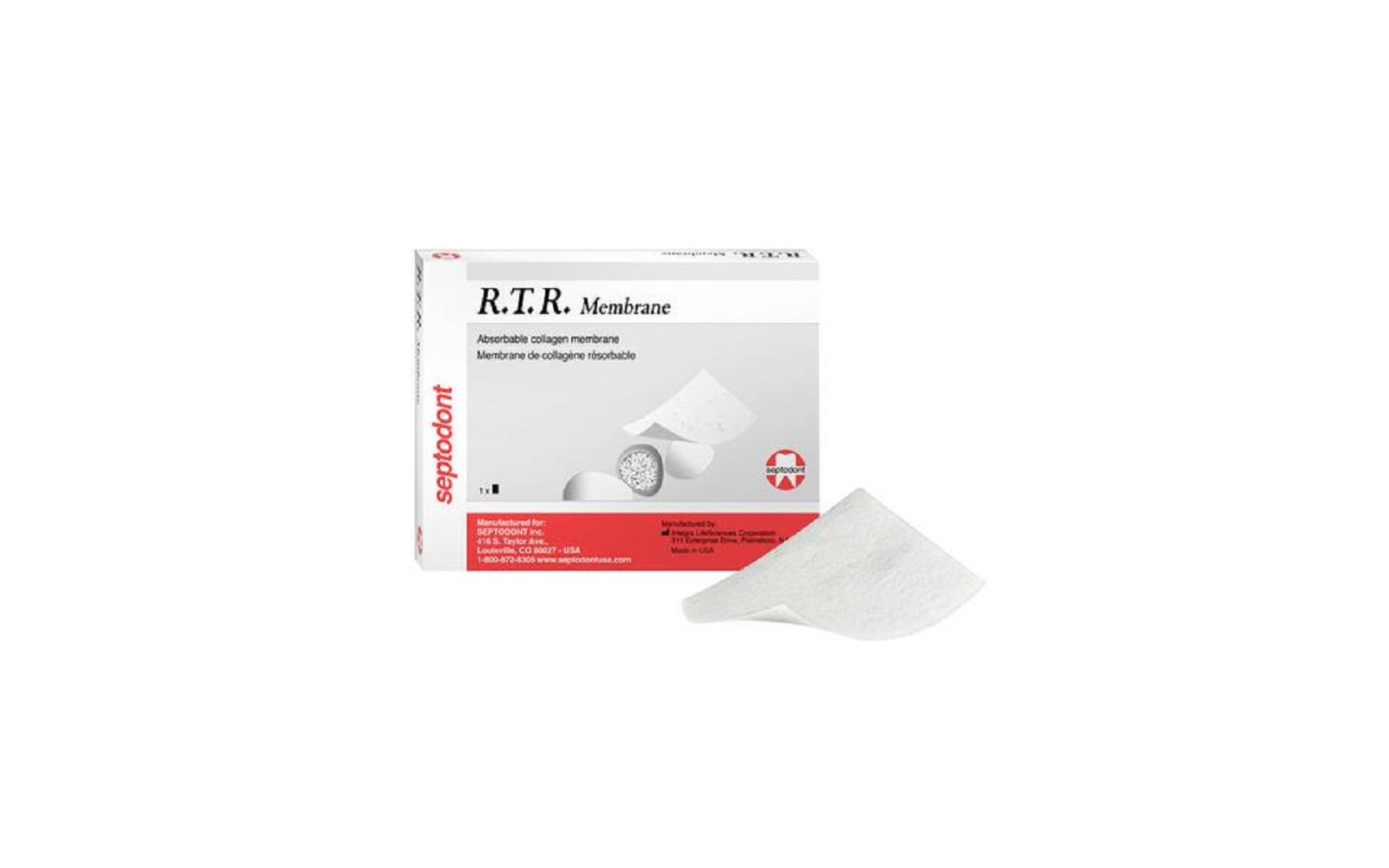
Product Description
R.T.R. Membrane by Septodont is a resorbable collagen membrane designed to support guided bone and tissue regeneration. Made from bovine collagen, it offers flexibility, strength, and easy adaptability to surgical sites, making it ideal for use with R.T.R. bone regeneration products.
Product Overview
This membrane can be easily trimmed to fit a variety of grafting sites and contours well to both hard and soft tissues. Its tear-resistant properties ensure stability during placement, while maintaining the barrier function required for predictable regenerative outcomes.
Key Features
- Made from biocompatible bovine collagen
- Tear-resistant yet flexible for easy handling
- Easily cut to size for site-specific applications
- Designed for use with R.T.R. bone regeneration products
- Maintains space and stabilizes the graft during healing
Common Applications
- Guided bone regeneration (GBR)
- Guided tissue regeneration (GTR)
- Socket preservation and ridge augmentation
- Use in conjunction with bone grafts, especially R.T.R. products
Technical Specifications
- Product Type: Resorbable collagen membrane
- Material: Bovine collagen
- Features: Flexible, tear-resistant, cut-to-fit
- Compatibility: R.T.R. bone regeneration products
- Manufacturer: Septodont Inc.
Customer Ratings
Average Rating: 4.7 out of 5 (based on clinical feedback)
Customer Feedback
- Easy to place and adapt
- Holds up well during suturing
- Excellent compatibility with bone grafts
- Trusted for reliable healing outcomes
8. CollaShield™ Collagen Membrane by Surgical Esthetics

Product Description
CollaShield™ Collagen Membrane by Surgical Esthetics is a fully resorbable, cell-occlusive membrane designed to support osteogenesis and protect graft sites during healing. Ideal for socket preservation, guided bone regeneration (GBR), and guided tissue regeneration (GTR), it provides structural integrity and biological compatibility.
Product Overview
Constructed from purified porcine peritoneum, CollaShield™ is engineered to inhibit unwanted cellular growth while maintaining the ideal environment for bone regeneration. The membrane resorbs naturally in approximately 16 weeks and can be easily trimmed to adapt to various clinical applications.
Key Features
- Fully resorbable in approximately 16 weeks
- Made from purified porcine peritoneum
- Cell-occlusive to inhibit epithelial cell migration
- Supports osteogenesis and graft site stability
- Easily trimmed to fit site-specific needs
Common Applications
- Extraction socket preservation
- Guided bone regeneration (GBR)
- Guided tissue regeneration (GTR)
- Graft site protection in periodontal and implant procedures
Technical Specifications
- Product Type: Resorbable collagen membrane
- Material: Purified porcine peritoneum
- Resorption Time: ~16 weeks
- Structure: Cell-occlusive
- Trimmable: Yes
- Manufacturer: Surgical Esthetics
Customer Ratings
Average Rating: 4.9 out of 5 (based on clinical feedback)
Customer Feedback
- Excellent for maintaining graft stability
- Easy to handle and contour
- Predictable resorption and healing support
- Trusted in both periodontal and implant surgeries
9. HeliMend Advanced Collagen Membrane by Miltex by Integra

Product Description
HeliMend™ Advanced Collagen Membrane by Miltex (Integra) is a long-lasting, absorbable membrane made from purified bovine tendon. It is designed to support guided tissue regeneration (GTR) by stabilizing wounds and maintaining space during healing in complex procedures requiring extended resorption time.
Product Overview
With an absorption period of approximately 18 weeks, HeliMend™ Advanced offers extended barrier function to support optimal tissue and bone regeneration. Its pliable form adapts easily to graft sites while maintaining mechanical integrity for predictable clinical outcomes.
Key Features
- Made from purified bovine tendon
- Long resorption time: approximately 18 weeks
- Stabilizes wound and maintains space during healing
- Ideal for guided tissue and bone regeneration procedures
- Fully absorbable—no need for surgical removal
Common Applications
- Guided tissue regeneration (GTR)
- Guided bone regeneration (GBR)
- Socket preservation and ridge augmentation
- Periodontal and peri-implant defect coverage
Technical Specifications
- Product Type: Absorbable collagen membrane
- Material: Purified bovine tendon
- Absorption Time: ~18 weeks
- Sterility: Sterile
- Manufacturer: Miltex by Integra
Customer Ratings
Average Rating: 4.8 out of 5 (based on professional feedback)
Customer Feedback
- Excellent strength and handling
- Reliable for extended regenerative procedures
- Predictable resorption profile
- Highly effective in maintaining space for healing
10. R.T.R.+ Synthetic Biphasic Bone Grafting Substitute Syringe by Septodont Inc
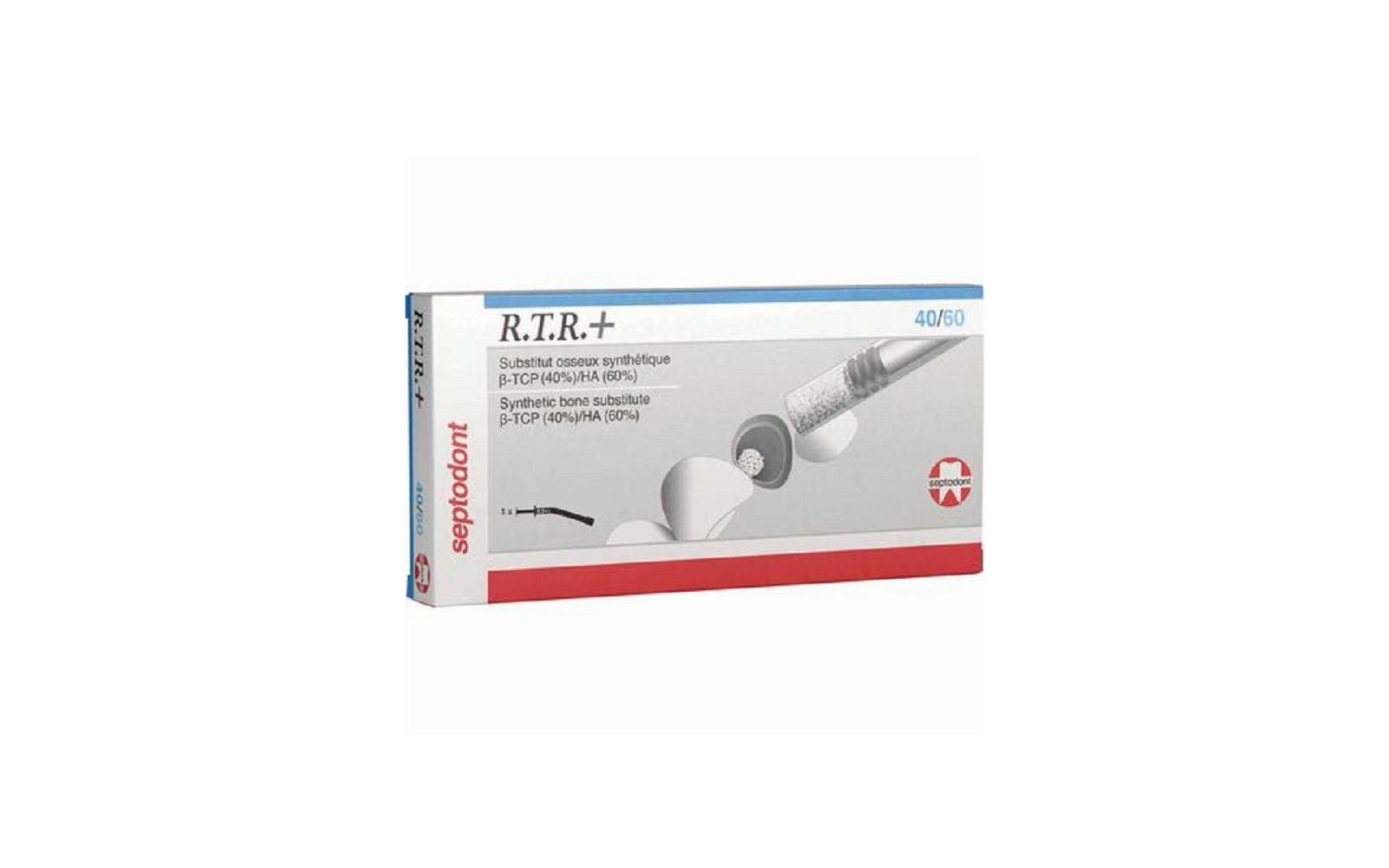
Product Description
R.T.R.+ Synthetic Biphasic Bone Grafting Substitute Syringe by Septodont Inc is a fully absorbable, sterile grafting material engineered for maxillofacial bone regeneration. Formulated with a biphasic combination of ß-Tricalcium Phosphate (ß-TCP) and Hydroxyapatite (HA), it supports effective bone healing and structural restoration across a wide range of surgical applications.
Product Overview
R.T.R.+ is offered in two biphasic ratios—40/60 and 80/20—to match specific clinical timelines for resorption and bone remodeling. Its micro- and macroporous structure enhances cellular colonization and vascular in-growth, while the osteoconductive composition creates a strong matrix for guided bone formation.
Key Features
- Synthetic, sterile, and fully absorbable biphasic formula
- Combines ß-Tricalcium Phosphate (bioactive) with Hydroxyapatite (stable)
- Micro-/macroporous architecture promotes cellular in-growth
- Osteoconductive matrix ideal for strong, guided bone regrowth
- Supplied in a ready-to-use syringe for easy handling and application
- Two resorption profiles:
- 40/60 (40% ß-TCP / 60% HA): Bone formation in 3–9 months
- 80/20 (80% ß-TCP / 20% HA): Bone formation in 9–12 months
Common Applications
- Post-extraction socket grafting
- Sinus lifts and ridge augmentations
- Cystic cavity fillings
- Periodontal and peri-implant defect repair
- Intraosseous and maxillofacial reconstruction
Technical Specifications
- Product Type: Synthetic biphasic bone graft substitute
- Composition: ß-Tricalcium Phosphate and Hydroxyapatite
- Forms Available: 40/60 or 80/20 ratios
- Application: Syringe format
- Sterility: Sterile, single-use
- Manufacturer: Septodont Inc.
- Resorption Time:
- 40/60: 3–9 months
- 80/20: 9–12 months
Customer Ratings
Average Rating: 4.9 out of 5 (based on clinician feedback)
Customer Feedback
- Easy to handle and apply with syringe delivery
- Excellent bone regeneration and scaffold support
- Customizable resorption timeline fits various clinical needs
- Reliable choice for advanced grafting procedures